Energy Storage
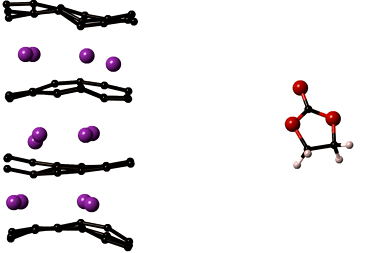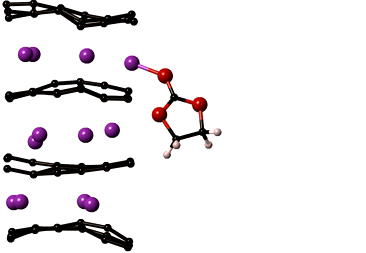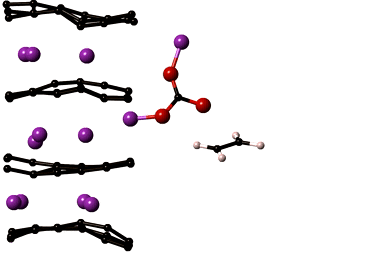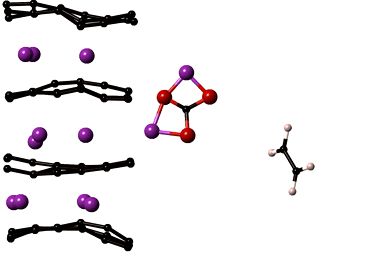
LiMn2O4 is the most sought after cathode material for automotive applications. In spite of its high capacity it is prone to a number of degradation mechanisms leading to reduced battery life. The most critical of which is manganese dissolution resulting in loss of cathode material and thereby the overall cell capacity. The key to improving the performance of LiMn2O4 is to understand the reactions that lead to manganese dissolution, which can then be used identify methods to increase the cathode stability.
Our lab develops computational models that capture reactions at the cathode surface which will help reveal chemical composition of the cathode surface and the mechanisms of manganese dissolution. Specifically, the reactive force field, ReaxFF, is being optimized for manganese containing electrochemical systems. ReaxFF will yield insights into the chemistry of regions that cannot be probed experimentally. These insights are necessary to develop chemically stable cathode materials prolonging the life of the cathode. The reactive force fields and DFT property compilations developed in the project will be valuable additions to the body of knowledge on interatomic potentials used by research in many disciplines of science and engineering.